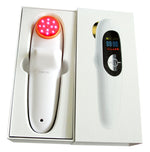
-
Description
-
Review
-
Shipping
-
Return
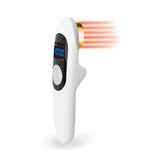
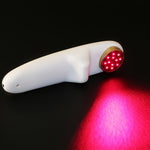
Product Principle
Our machine using 808nm for medical use and 650nm for household use, through specific parts of irradiation and using light radiation of laser and wavelength relation to change the biological characteristics as well as directly penetrate the meridian,the non-thermal photons of light that are emitted from the laser pass through the skins layers (the dermis, epidermis, and the subcutaneous tissue or tissue fat under the skin)
When cells absorb this light energy, it initiates a series of events in the cell that is theorized to eventually result in normalizing damaged or injured tissue, a reduction in pain, inflammation, edema and an overall reduction in healing time by increasing intracellular metabolism.

Product Application
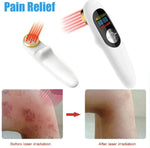
1. People and animals pain relief, knee arthritis, rheumatoid arthritis, athletic system injuries, soft tissue injuries, relieve muscle and joint pain.
2. Diminish inflammations, reduce swelling, wounds & ulcers, osteoarthritis, acupuncture, rehabilitation therapy.Knee, Feet, Ankle, Shoulder, Back, Elbow, Hand, Joint & Muscle...etc Arthritis Tennis Elbow Tailbone Inflammation




Product Specification
|
Laser Wavelength |
650nm+808nm |
|
Laser Operating Voltage |
110-240 V |
|
Timing Range |
10-30mins for adjustment |
|
Environment Temperature |
-20°C-40°C |
|
Relative Humidity |
≤85% |
|
Atmospheric Pressure |
86kpa-106kpa |
|
Power Source Use |
Lithium battery with capacity of 1000 mAh |
|
Warranty |
one year |


Package includes:
1x Pain relief low-level cold laser device
1x Power adapter
1x USB line
1x Manual
Instructions
1.Distance: Apply the light from 1-2 inches to the skin.
2.Session Time: Please use 2 times a day for about 30 minutes for each time. 7-10 days for one treatment course. After 7-10 days, stop one or two days. Then, continue for next course. No side effects under the long-term use. Make sure to stay hydrated during the treatment.
DISCLAIMER:
This product is not intended to diagnose, cure or prevent disease. LASTEK® makes no claims, representations or warranties regarding the ability of this product to cure any physical, skin or mental condition. A qualified health professional should be consulted with regard to any condition requiring medical attention. This device is intended only for personal household use, commercial use is not intended. As a therapeutic device, LLLT has achieved a FDA low-risk general wellness status.
For orders placed before 7am AEDT, we endeavour to process the same business day. Orders placed after 11am AEDT will be processed the next business day.
During sale events and new collection launches, there may be a slighly longer processing time.
All Auguste orders are hand-picked and packed with love from Byron Bay, Australie.
You can choose between a refund or a credit note on full priced items.
- Item(s) must be returned in their original condition and packaging: unworn, unwashed and with all tags attached.
- Earrings cannot be returned due to health and safety reasons.
- Return shipping methods and associated costs are the responsibility of the customer.
- Sale items can not be refunded for change of mind.